Which Statement Describes the Bonds in Nitrate
A The energy absorbed for bond breaking is greater than the energy released by bond formation. The π -bond electrons are delocalized over the three NO bonds.

Solved Which Of These Statements Describe The Bonding In The Chegg Com
Because Iron is transition metal while S is a non-metal.
. Which statement correctly describes X and Y. Up to 24 cash back 22Magnesium nitrate contains chemical bonds that are ACuO BCuO2 CCu2O DCu2O2 23Which formula represents copperI oxide. Which of the following statements correctly describe bond energy.
Bond energies are shown. Bond 1 3 1 1 3 1 1 3 2 2 3 2 3 133 The bond between nitrogen and oxygen in the nitrate anion is somewhere between a single bond and a double bond but closer to a single bond. Hreactants Hproducts 3.
A 1192 B 694 C 694 D 1192 13 Which statements about hydrogen fuel cells are correct. A Nitrogen and oxygen have an electronegativity difference of 05 so the bond is polar covalent with oxygen pulling the electrons toward it. 12 Which statement describes an exothermic reaction.
The bonds strongly hold ions together reducing. 24 A student heated the carbonates and nitrates of sodium and copper. Compound heated gases.
Because oxygen has a greater electronegativity than hydrogen science. The resonance is shown in the figure below. Choose the statement that correctly describes the relationship between the two ions in terms of bond length and bond energy.
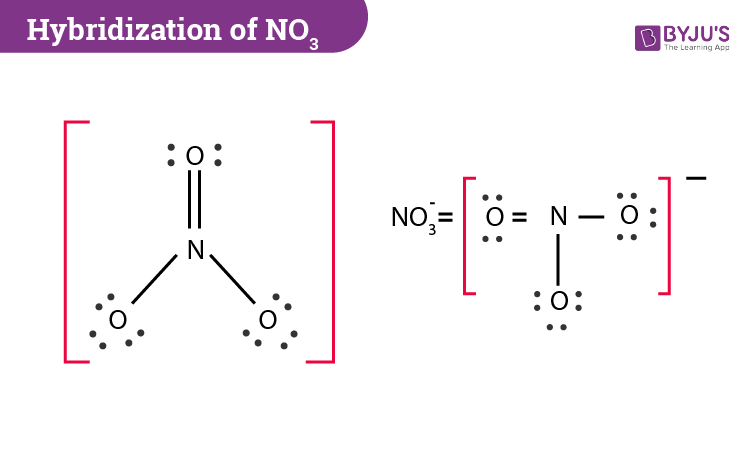
Which statement describes a reaction of potassium hydroxide. The results are shown. Lewis diagrams for the nitrate and nitrite ions are shown below.
A water molecule is held together by two single polar covalent bonds. B Fe and S have a covalent bond and S and O have covalent bonds too. The bond between the atoms in the molecule is AH Br BH Cl CI Br DI.
The energy consumed when chemical bonds are broken during a chemical reaction C. Which of these statements describe the bonding in the nitrate ion. The total energy required to break apart a molecule depends on the number and type of bonds.
Which of the following phrases best describes the term homogeneous. Nitrite has shorter and stronger bonds than nitrate. The energy released when chemical bonds are formed during a chemical reaction B.
Bond bond energy in kJ mol CH 410 CO 805 OH 460 OO 496. D sodium nitrate 19 Potassium hydroxide is a base. Describe the bonding in ethylene C2H4 in terms of valence bond theory.
Lewis diagrams for the nitrate and nitrite ions are shown below. D X is harder and stronger than Y. There are three o bonds one is formed by the overlap of sp2 hybrid orbitals on N O.
C X is a solid and Y is a liquid. Hreactants Hproducts D. There are two bonds lengths where the bonds oscillate between 122 pm and 136 pm.
Bond bond energy in. Which one of the following statements best describes the enthalpy change of a reaction. Chemistry 26102021 1650 SkinnestXOXO.
The difference between the energy released by. A Nitrogen and oxygen have an electronegativity difference of 05 so the bond is polar covalent with oxygen pulling the electrons toward it. This is called electron delocalization.
Uniform composition throughout b. Bond bond energy in kJ mol CH 412 HO 463 CO 743 OO 498 What is the overall energy change in kJ mol for the above reaction. Describe the bonding in the nitrate ion NO3- in terms of delocalized molecular orbitals.
The bond energies are shown in the table. Ammonium nitrate is added to water at 25 C and the mixture stirred. Bond energy values are always positive.
Which statement describes the bonds in nitrate NO3-. Mixed but will separate over time e. Two of the above statements are valid.
There are two bonds lengths where one bond is 136 pm and the other is 122 pm. Correct answer to the question Which statement describes the bonds in nitrate NO3-. The bonds prevent ions from moving throughout the crystal so a solid ionic compound is a poor conductor.
Chemistry questions and answers. Metroplex Corporation will pay a 304 per share dividend next year. Two liquids that are mixed together.
Which statement best describes the bond length s in the nitrate ion shown. Aelectrovalent Bionic Cnonpolar covalent Dpolar covalent 24Two atoms of element A unite to form a molecule with the formula A2. B Nitrogen and oxygen have an electronegativity difference of 05 so the bond is nonpolar covalent.
These are true and false questions and I want to make sure that I got them right. A X is a pure metal and Y is a. Metallic bonds are responsible for many properties of metals such as conductivity.
The bond energies are shown in the table. B The energy absorbed for bond breaking is less than the energy released by bond formation. Not a uniform composition throughout d.
How do ionic bonds affect the properties of ionic compounds. Monomers have a CC double bond. Image is not available to copy A Nitrite has longer and stronger bonds than nitrate.
Which statement describes what happens in this reaction. The statement that describes the bonds in FeSO₄ is. A Nitrogen and oxygen have an electronegativity difference of 05 so the bond Which statement describes the bonds in nitrate NO3-.
Select all that apply There is one o bond formed by the overlap of two p orbitals. All three of the above statements are valid. Nitrite has longer and weaker bonds than nitrate.
Two are formed by overlap of an sp3 hybrid orbital on N an sp2 hybrid orbital on O. Choose the statement that correctly describes the relationship between the two ions in terms of bond length and bond energy. A Nitrogen and oxygen have an electronegativity difference of 05 so the bond is polar covalent with oxygen pulling the electrons toward it.
The bond that forms between a Fe iron and S sulfur is a covalent bond. B Nitrite has longer and weaker bonds than nitrate. The bond length of N-O is 136 pm and NO is 122 pm.
C The energy released by bond breaking is greater than the energy absorbed for bond formation. The bonds weakly hold ions together increasing the melting point. Due to resonance all bond lengths would be the same Share Improve this answer answered Dec 2 2018 at 1523 Aditya Garg 457 4 13 Add a comment.
Covalent bonds are also found in the bonding of S and O because they are both Non-metals. Mixed but can be separated c. Describe the bonding in ethylene C2H4 in terms of valence bond theory.
NO3-nitrate PO43-phosphate SO42-sulfate SO32-sulfite. Dur to resonance none of the cannonical structures explain the bond length in nitrate except the resonance hybrid which happens to be the last drawn structure. Nitrite has longer and stronger bonds than nitrate.
The compound that has the smallest ions with the least charge.
Chemical Forums What Does The Structure Of A Nitrate Ion Look Like

Hybridization Of No3 Hybridization Of N And O In Nitrate
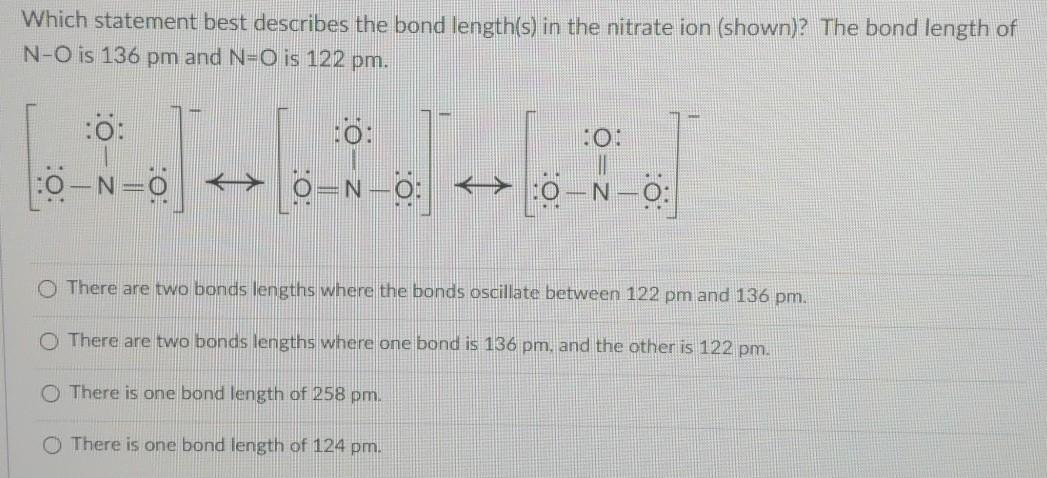
Solved Which Statement Best Describes The Bond Length S In Chegg Com
Comments
Post a Comment